
Some of this video, we're gonna go over Question 46 from Chapter, For which says supposed 50 milliliters of 500.25 Mueller cobalt chloride solution is added to 25 milliliters of 250.35 Mueller nickel to chloride solution.

And we dilute that to 100 so that I will have a concentration of 0.0 Fix for zero Moller hcl. And then we have 10 mL or taken 10 mL of our solution A. We're gonna multiply that I do something wrong here. But we're gonna do that right now for part A. That should be v times m equals v times m. The volume, the initial volume times the initial polarity equals the final volume times There should be a times right there.Ĭhina equals V f.
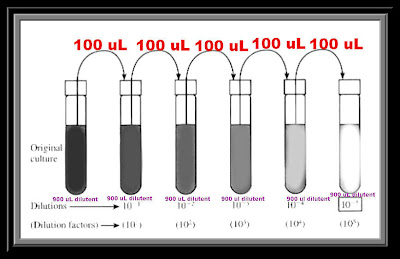
I believe that's everything we need to solve the problem in the wonderful world of science. Our task is to find the concentration of the solution in part, beat concentration of final solution. Then we take 10 mils of solution A and we dilute that again to 100 mils in a flute in a volumetric flask. I couldn't fit my l in there we take a 25.0 mill leader, al a quart of that solution and we dilute it to 100.0 mL in a 100 mil volumetric flask. Everyone's well were given 250 mil eater milliliters of a 0.136 Mohler solution of hydrochloric acid. So I'm gonna call it cereal Delusions Light. Here's a serial delusions problem, and I just wanted to say serial delusions because I think it sounds cool.


 0 kommentar(er)
0 kommentar(er)
